Since January 1st 2025, MultiMed Engineers participates in the ENKORE Project — Propelling the shift toward the future of circular, safe and sustainable packaging and single use device ecoDesigned solutions through healthcare environments.
The healthcare sector is hindered by several barriers that hamper the application of circular economy principles (e.g. the safety restrictions of the domain limit the use of recycled materials due to the need of materials biocompatibility, and safety in products to be used in the human body).
Led by a multidisciplinary consortium of 39 partners (plus 13 industry affiliates) from 15 EU countries plus UK and USA, ENKORE aim to tackle challenges through a modular concept represented in the Figure below, and develop an ecoDesign framework that supports the development of safe and environmental compliant devices eco-responsible packaging, which minimize the environmental impact, reduce the carbon footprint, and maximize the use and preservation of resources.
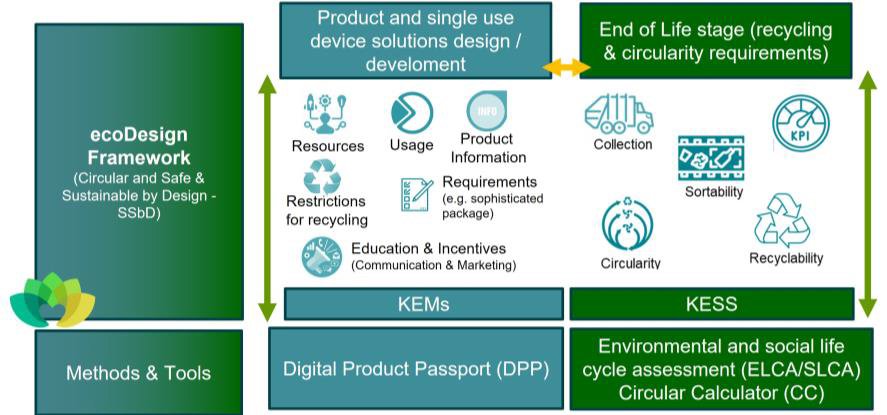
The ENKORE modular concept
The main goal is to connect the design of the medical devices packages with the end-of-life stage, thus the technologies that support circularity are taken into account at the medical device conception stage.
ENKORE framework will be validated in 5 Reference Use Cases (RUCs), led by 5 different health regions that bring HPCs and policy maker, 3 large EU hospitals and the reference network for European Regional and Local Health Authorities (EUREGHA). The project developments and RUCs are supported by several associations and NGOs, a packaging manufacturer and a group of SMEs and researchers, specialists in circularity, LCA, social sciences, environment, circularity, and materials.
The validation of the framework shall provide evidence to work with policymakers, creating new or revised standards and create tangible/quantitative evidence. Policy making and regulatory engagement will be strongly performed. The methods and tools comprise Environmental and Social Life Cycle Assessment (ELCA/SLCA), Circularity Calculator (CC) and Digital Product Passport (DPP) approaches, which could be adapted during the second stage of the proposal.
The Project Consortium is coordinated by UNIVERSIDAD POLITECNICA DE MADRID (ES) and includes — in addition to MultiMed Engineers — the following Partners: MEDTRONIC IBERICA SA (ES), SOFRADIM PRODUCTION SASU (FR), PREDICTBY RESEARCH AND CONSULTING S.L. (ES), UNIVERSITA CAMPUS BIO MEDICO DI ROMA (IT), UNIVERSITEIT LEIDEN (NL), POLITECHNIKA POZNANSKA (PL), INSTITOYTO BIOIATPIKHE TEXNOLOGIAS (EL), CHARITE – UNIVERSITAETSMEDIZIN BERLIN (DE), FONDAZIONE POLICLINICO UNIVERSITARIO CAMPUS BIO MEDICO (IT), UNIVERSYTET MEDYCZNY W LODZI (PL), CONSORCIO MAR PARC DE SALUT DE BARCELONA (ES), TWI ELLAS ASTIKI MI KERDOSKOPIKI ETAIREIA (EL), FUNDACION UNIVERSIDAD FRANCISCO DE VITORIA (EL), SAMENWERKENDE TOPKLINISCHE OPLEIDINGSZIEKENHUIZEN (NL), OSAI AUTOMATION SYSTEM SPA SOCIETA’ BENEFIT (IT), AGENZIA REGIONALE PER LA SALUTE ED IL SOCIALE (IT), EUROPEAN REGIONAL AND LOCAL HEALTH AUTHORITIES ASBL (BE), NAUCNOISTRAZIVACKI INSTITUT VERLAB ZA BIOMEDICINSKI INZINJERING MEDICINSKE UREDAJE I VJESTACKU INTELIGENCIJU (BA), ENOSI ASTHENON ELLADAS (EL), STERIMED HOLDING (FR), SERVICIO MADRILENO DE SALUD (ES), IDRYMA TECHNOLOGIAS KAI EREVNAS (EL), HAPPY MONDAYS COMMUNICATION SL (ES), PFIZER INC (US), NOVO NORDISK A/S (DK), ELI LILLY AND COMPANY (US), JOHNSON & JOHNSON MEDICAL GMBH (DE), TAKEDA PHARMACEUTICALS INTERNATIONAL AG (CH), FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH (DE), DUPONT DE NEMOURS (LUXEMBOURG) SARL (LU), BOEHRINGER INGELHEIM INTERNATIONAL GMBH (DE), INTERNATIONAL SOLID WASTE ASSOCIATION (NL), FORUM DES PATIENTS EUROPEENS (BE), BAXTER R AND D EUROPE (BE), ACTIVE AGEING ASSOCIATION (ES), UDG ALLIANCE (CH), WORLD RESOURCES FORUM ASSOCIATION (CH), PFIZER LIMITED (UK), Pfizer R&D UK Limited (UK), PFIZER IRELAND PHARMACEUTICALS (IE), Pfizer Manufacturing Deutschland GmbH mit Sitz Illertissen (DE), Pfizer Italia s.r.l. (IT), ELI LILLY AND COMPANY LTD (UK), Lilly Deutschland GmbH (DE), Eli Lilly Danmark A/S (DK), BOEHRINGER INGELHEIM PHARMA GMBH & CO KG (DE), Boehringer Ingelheim Pharmaceuticals, Inc. (US), DePuy International Limited (UK), Johnson & Johnson Services, Inc. (US), TECHNOVATIVE SOLUTIONS LTD (UK).
MultiMed Engineers, is involved in the following project Work-packages:
- WP1 Meta-assessment, evidence identification, exchange of knowledge and requirements, where it will work on Task 1.5 Reference Use Cases stakeholders’ and local sites` requirements refinement and planning
- WP4 Reference Use Cases and Validation, where it will work on Task 4.1. Design the Reference Use Cases, evidence and KPI definition for the use cases, Task 4.2 Tools, technology services and protocol deployment, Task 4.4 Local pilot sites continuous management, transversal support, and validation and Task 4.5 Fit assessment between stakeholders’ values, requirements and developed tools, methods, and models
- WP6 Communication, dissemination and results exploitation and sustainability, where it will work on Task 6.1 Dissemination and communication strategy, plan and monitoring, Task 6.2 Exploitation and financial sustainability strategy and Task 6.4 Drivers and solving barriers in adopting and implementing evidence-based interventions
- WP7 Project Coordination, Administration, and management, where it will contribute with the other partners to the smooth conduction of the project activities, from both the technical and administraive perspective.
Project Website: https://enkoreecohealthcare.eu/
 This project has received funding from the European Union’s Horizon Europe research and innovation programme and the contributing partners of the Innovative Health Initiative Joint Undertaking, under grant agreement No 101166707
This project has received funding from the European Union’s Horizon Europe research and innovation programme and the contributing partners of the Innovative Health Initiative Joint Undertaking, under grant agreement No 101166707
 MultiMed Engineers
MultiMed Engineers